Abstract
Background:
Chimeric antigen receptor (CAR) T cells targeting CD19 have achieved great clinical responses in patients with relapsed or refractory acute B lymphoblastic leukemia (R/R B-ALL). However, severe adverse events such as cytokine release syndrome (CRS) and neurotoxicity restrict it to further application. Tocilizumab against human interleukin-6 (IL-6) receptor is a common treatment for CAR-T cell therapy associated cytokine release syndrome. Corticosteroids are used when remission is not reached after the application of tocilizumab as well as neurotoxicity occurs, according to the guidance. However, their suitable timing still remains unclear when taking their efficacy and side effects into consideration.
Methods:
From January 2016 to July 2020, in our phase 1/2 clinical trials (NCT02965092、NCT04008251), 55 patients with R/R B-ALL were enrolled and injected with anti-CD19 CAR-T cells. Clinical laboratory tests on day 0、4、7、10、14、21、28 after infusion as well as endpoints、adverse events and treatment were recorded.
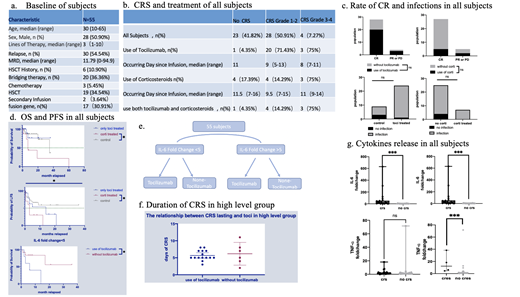
CRS and neurotoxicity were graded according to American Society for Transplantation and Cellular Therapy (ASTCT),and infection severity was classified as mild, moderate, severe, life-threatening, or fatal. (Young et al. Biol Blood Marrow Transplant 2016; 22:359-70.)
Patients were assigned to four cohorts based on the fold change of IL-6 and the use of Tocilizumab. We defined fold change as the ratio of peak before Tocilizumab given to baseline in Tocilizumab group and the ratio of peak within 28 days to baseline in non- Tocilizumab group. According to the statistics, two groups were separated into high level (fold change over 5) and low level (fold change below 5), respectively.
Wilcoxon tests、Log-rank tests and Fisher's exact tests were used to analyze statistics in GraphPad Prism 9.
Results:
During the observation period of 28-day-postinfusion, the use of Tocilizumab or corticosteroids did not significantly reduce the response rate or increase infectious risk (P>0.99, P=0.052). Doing a median follow-up of 7 months, the use of corticosteroids was significantly associated with shorter overall survival (OS) and progression-free survival (PFS), while it did not appear when Tocilizumab was applied alone. In addition, significantly fold change of IL-6, IL-10 were observed among subjects suffering cytokine release syndrome before the use of Tocilizumab or corticosteroids and higher levels of TNF-α were observed in 3 subjects with mild neurotoxicity (P=0.0002, P<0.0001, P=0.0004).
In high level group, patients treated with Tocilizumab had mild CRS limiting to grade 1-2, with shorter duration of CRS (median=5) than non-Tocilizumab (median=6) , though it is without significant difference (P=0.874).
In low level group, the use of Tocilizumab is associated with shorter PFS(P=0.0275)as well as severe cytokine release syndrome. Two patients developed grade 4 CRS after infusing Tocilizumab,with apparently increased level of IL-10 (fold change=200) or IFN-γ (fold change=114.24).
Neurotoxicity occurred in four patients in Tocilizumab group, and their IL-6 levels increased significantly after treatment, reaching an average peak of 1000pg/ml (157-22001.9). No neurotoxicity were observed in non-Tocilizumab group.
Conclusion:
Our study demonstrate that severe and persistent CRS could be avoided by applying Tocilizumab when IL-6 has increased over 5-fold from baseline. Tocilizumab is not recommended to use with little change of IL-6 because it fails to suppress the inflammatory response, and may trigger the activation of other cytokines and accelerate the progress of disease recurrence in patients.
Although corticosteroids were associated with relapse, we still suggested that corticosteroids should be administrated to antagonize neurotoxicity with symptoms and significantly increased IL-6 levels after the infusion of Tocilizumab.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal